 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
Member
AdOo
Join Date: Mar 2016
Location: Bordeaux
Posts: 91
Rep Power: 10  |
Hi all,
I would like to change the gas in the solver XiFoam, (running methane instead of propane). The thing is, I don't know how to calculate the Janaf coefficient for gas mixture. Does anybody know ? Another question more specific about this solver I think, WHY is there nMoles = 1 for products, whereas it's set to 24.8095 for reactants (in the combustionProperties file). Where does this 24.8095 comes from ? Thanks a lot, hopping one of you hold the answer, Adrien |
|
|
|

|
|
|
|
|
#2 |
|
Member
AdOo
Join Date: Mar 2016
Location: Bordeaux
Posts: 91
Rep Power: 10  |
Ok, about the gas mixture,I've found my answer, using the Dalton's law in order to determine the janaf coeff of a gas mixture.
https://en.wikipedia.org/wiki/Dalton%27s_law Leading to : Cp = 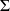 xi . Cpi where xi is the mole fraction. xi . Cpi where xi is the mole fraction.Unfortunately, I can't handle why there's nmoles = 24.8095 for reactants and nmoles = 1 for products...(According to me, they all should be at 1...) If someone have the answer, let me know. Have a nice ay, Adrien |
|
|
|

|
|
|
|
|
#3 |
|
Member
Join Date: Feb 2014
Posts: 63
Rep Power: 12  |
Hi adrieno,
To change the cp coefficients you can use the adiabaticFlameT utility. cd $FOAM_UTILITIES/thermophysical/adiabaticFlameT adiabaticFlameT controlDict you ll get values for the fuel specified in controlDict. However I have the same problem about 'nmoles' could not find an answer. If you find an answer please share. |
|
|
|

|
|
|
|
|
#4 |
|
Member
AdOo
Join Date: Mar 2016
Location: Bordeaux
Posts: 91
Rep Power: 10  |
Hi Uyan,
I don't understand how to use the controlDict you've told me about : Code:
/*--------------------------------*- C++ -*----------------------------------*\ | ========= | | | \\ / F ield | OpenFOAM: The Open Source CFD Toolbox | | \\ / O peration | Version: 2.2.2 | | \\ / A nd | Web: www.OpenFOAM.org | | \\/ M anipulation | | \*---------------------------------------------------------------------------*/ FoamFile { version 2.0; format ascii; note "settings for calculating the adiabatic flame temperature"; class dictionary; object controlDict; } // * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * // P 1e5; T0 300.0; H2 { fuel H2; n 0; m 2; } CH4 { fuel CH4___ANHARMONIC; n 1; m 4; } ETHYLENE { fuel C2H4; n 2; m 4; } PROPANE { fuel C3H8; n 3; m 8; } OCTANE { fuel C8H18(L)_isooctane; n 8; m 18; } N-HEPTANE { fuel C7H16_n-heptane; n 7; m 16; } $H2; // $CH4; // $PROPANE; // ************************************************************************* // Another question : Do you know how to adapt the coeff in the combustionPorperties file ? for another gas like methane in my case (I just handle the laminar speed with Su but I'm a bit lost with all the other coefficients) Thanks a lot, Adrien |
|
|
|

|
|
|
|
|
#5 |
|
Member
Join Date: Feb 2014
Posts: 63
Rep Power: 12  |
Hi Adrian,
Type these two commands on an OpenFOAM terminal, Code:
cd $FOAM_UTILITIES/thermophysical/adiabaticFlameT adiabaticFlameT controlDict this will print out cp coefficients you need for the fuel specified in the controlDict. In the controlDict you have shown here it is H2. Well for other values in combustionProperties dictionary you ll have to look for values from literature and try them out. |
|
|
|

|
|
|
|
|
#6 |
|
Member
AdOo
Join Date: Mar 2016
Location: Bordeaux
Posts: 91
Rep Power: 10  |
Thank you very much for your time Uyan,
Does it gives the Cp value for a stoechiometric mixture ? (This is what I'm looking for). And just to know, is it possible to have it for another stoechiometric value ? For the other coefficients, this is what I was trying to do... but it's hard to find especially as the Weller combustion model doesn't seem very common. Even the laminar flame speed seems to differ from a revue to another ! Aaaa combustion is such a complex science for neophyte ! Thank you again Uyan. |
|
|
|

|
|
|
|
|
#7 |
|
Member
Join Date: Feb 2014
Posts: 63
Rep Power: 12  |
Hi Adrian,
For you and others who wondered why the "nMoles" is not equal to 1 in the reactants side but 24.8. I finally found the answer how to calculate. So I post it here for anyone who is stuck there. In the XiFoam tutorial file (moriyoshiHomogeneous), thermophysicalProperties dictionary contains properties for reactants and products as following. Code:
reactants
{
specie
{
nMoles 24.8095;
molWeight 29.4649;
}
.....
Code:
products
{
specie
{
nMoles 1;
molWeight 28.3233;
}
Reactants ---------------> Products  For reactants   For products it is considered as one species, so nMoles = 1 |
|
|
|

|
|
|
|
|
#8 |
|
Member
AdOo
Join Date: Mar 2016
Location: Bordeaux
Posts: 91
Rep Power: 10  |
Thank you Uyan, that's what I've done finally in my simulation, but that was just a guess finding the way the weller combustion model was working...
|
|
|
|

|
|
 |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| compile of new combination of property in thermophisicalpropery | milad653279 | OpenFOAM Running, Solving & CFD | 4 | April 6, 2017 05:35 |
| Janaf (Old ?) coefficient | adrieno | OpenFOAM Pre-Processing | 1 | April 18, 2016 08:07 |
| Ansys CFX problem: unexpected very high temperatures in premix laminar combustion | faizan_habib7 | CFX | 4 | February 1, 2016 18:00 |
| Question about heat transfer coefficient setting for CFX | Anna Tian | CFX | 1 | June 16, 2013 07:28 |
| Automotive test case | vinz | OpenFOAM Running, Solving & CFD | 98 | October 27, 2008 09:43 |