 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
New Member
Maksim
Join Date: Jul 2020
Posts: 10
Rep Power: 6  |
Hello everyone!
I am trying to incorporate chemical reaction calculation in my compressible FVM solver, and there is a question occured about how to account heat of reaction. In most examples, where such effect is considered, energy equation is solved regarding enthalpy of a flow. But i use in my solver the HLLC scheme to calculate flows, thus for energy balance equation total energy is used. So i'm trying to find a way to calculate heat effect of reaction in terms of total or internal energy and not enthalpy. In chemical literature i found two variants of a heat effect of a reaction. Isochoric of reaction at constant volume and isobaric at constant pressure. They are expressed as 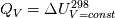 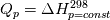 For ideal gas there relation is 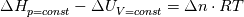 But the 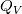 never described any further and never used in the texts, only never described any further and never used in the texts, only 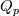 is, as reactions in laboratory chemistry mostly considered isobaric. is, as reactions in laboratory chemistry mostly considered isobaric.Additionaly to my mind comes the thought, that solving equation of chemical reaction in finite volume cells, we can consider each cell independently as a fixed volume reactor of ideal mixing, so reactions that flow in cell are actually not isobaric, but isochoric. So my question is, how should i calculate energy change due to chemical reaction? 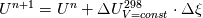 or 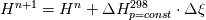 or maybe 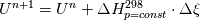 where 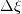 -- calculated number of reactions per volume on n step, -- calculated number of reactions per volume on n step, 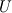 , , 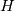 -- internal energy and enthalpy per volume correspondingly. -- internal energy and enthalpy per volume correspondingly.
|
|
|
|

|
|
|
|
|
#2 |
|
Senior Member
Lucky
Join Date: Apr 2011
Location: Orlando, FL USA
Posts: 5,761
Rep Power: 66    |
Lab experiments are most often isobaric because they mix their chemicals in a petri dish that's open to ambient. Using a combustion example, an isochoric reaction is what happens when you burn in a piston-cylinder engine with no stroke, i.e. a bomb.
Assuming your energy equation is indeed working properly except for the source term and has no bugs, consider that if you use the isochoric heat of reaction then you should use the constant volume specific heat (cv) in your formulation of total energy. If you use isobaric heat of reaction, then use constant pressure specific heat (cp). Now that would be an equation in Temperature which you are not interested in because you say you want internal energy, but the transport equations that you write down using all these different approaches must be consistent with one another. The actual flow process undergone by the fluid will be solved by listening to the transport equation, not our emotions. Real flows are neither isochoric nor isobaric. Isochoric heats and constant volume specific heats are both unpopular because most flows are not inside rigid tanks with closed ends and having to keep track of the boundary work that would occur all the time is tedious. Of course you can still do it (in your code for example) if you are willing to deal with the extra bookkeeping. Hint: you need to write down the entire energy transport equation and look at where the pressure work term is. Last edited by LuckyTran; June 2, 2023 at 11:39. |
|
|
|

|
|
|
|
|
#3 | |
|
New Member
Maksim
Join Date: Jul 2020
Posts: 10
Rep Power: 6  |
Quote:
I use division on processes in my work, so there is several energy equations, that are solved. On the advection stage i'm solving Euler equation for total energy, using HLLC: 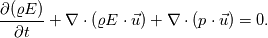 Next i consider various diffusive effects. There i substract kinetic energy from total and get internal energy, which express in terms of temperature: 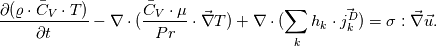 And for the chemical reaction stage, i guess, i can solve equation: 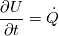 where: 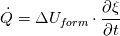 Is this approach plausible? |
||
|
|

|
||
 |
| Tags |
| chemical reaction, finite volume method, heat of reaction |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Turbulent heat flux | Eicosane | Main CFD Forum | 2 | April 24, 2023 14:07 |