 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
New Member
Jory
Join Date: Apr 2017
Location: Canada
Posts: 4
Rep Power: 9  |
Hello everyone,
I'm trying to add a decoupled finite-rate chemistry solver to a FEM compressible flow code. Right now we are getting incorrect steady-state chemical composition and we're pretty sure the issue is not with the source terms. I am having a bit of trouble figuring out the way the system should be set up, so if there are any experts in this field I would love to pick your brains. The equations to be placed in the decoupled chemistry system are the conservation of species mass (densities): 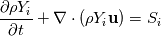 where S_i is the chemical source term, in [kg/m3.s]. The way I have things currently set up is as follows:
I think that this is not the best way of doing things because you essentially get a "new" nodal density from the flow solution, and a different "new" nodal density from the chemistry solution, and I'm not quite sure which one to plug back in to evaluate all of the new variables. I could solve the chemistry system using the "old" flow variables at time level n, but chances are the solved densities will not be the same anyway (due to computational error and the desire to be able to update the systems with different relaxation factors). I am being told by people to solve directly for the primitive variables (aka the mass fractions). However, I'm not sure how to do this. I have seen the following equation in a few places:  However I don't think this is correct, since in the absence of source terms the mass fractions should stay the same, correct? The same equation with the conserved quantity being the species concentration [mol/m3] makes more sense. Thanks for any help |
|
|
|

|
|
|
|
|
#2 |
|
Senior Member
Join Date: Jul 2009
Posts: 358
Rep Power: 19  |
I can't add much, but it is normal to solve n-1 species continuity equations along with the global continuity equation. The nth species density is given by the mixture density minus the n-1 species densities. Then you avoid having two mixture densities.
|
|
|
|

|
|
|
|
|
#3 |
|
Senior Member
Lucky
Join Date: Apr 2011
Location: Orlando, FL USA
Posts: 5,761
Rep Power: 66    |
You neglected the species diffusion term.
This is a species transport equation, it is not a chemistry equation. There is nothing here that describes the chemical reaction, in fact the chemistry is an input to this problem in the source term. Normally you label it R as a reaction rate and reserve S for external sources. The species transport equation only describes how the species flow or move around in the domain, it doesn't tell you anything about the reactions the species are undergoing. From the "chemistry equations" and by chemistry equations I mean the set of rate equations that comes from the kinetic model you get a reaction which is the S in the species transport equation. If you are using Arrhenius rate-laws, then this is a big system of coupled ode's that you would solve using some other specialized coupled ode solver. You solve the species transport equation to get a new mass fractions. So no you do not get two new mass fractions. |
|
|
|

|
|
 |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| Equilibrium Reaction Rates/Gas Chemistry | Greg Perkins | FLUENT | 6 | January 6, 2017 03:59 |
| The problem of Stiff Chemistry Solver in Multiphase model | shenzhou1987 | FLUENT | 1 | January 11, 2016 03:52 |
| Chemical Equilibrium Simulation using Fluent | enigma | ANSYS | 2 | October 27, 2011 09:02 |
| non-premixed combustion with equilibrium chemistry | Thiru | FLUENT | 1 | October 30, 2010 01:14 |
| Equilibrium chemistry in mixture fraction space | Peter | FLUENT | 2 | March 11, 2009 10:34 |