 |
|
|
[Sponsors] | |||||
|
|
|
#1 |
|
Senior Member
Sebastian Gatzka
Join Date: Mar 2009
Location: Frankfurt, Germany
Posts: 729
Rep Power: 20  |
Hello world.
I'm trying to simulate a solid reaction inside an ash particle. Therefore I am using the Discrete Phase Model (DPM). Nothing special so far, DPM works fine. Let's consider this: I want to apply the DPM on a converged simulation of a power plant. So I am using the DPM without interaction with the continuous phase. All variables inside the domain are solved, therefore I have the whole zoo of species concentrations at hand. The most important species to me is oxygen. Why? Because I want to simulate this reaction:  + 0.5 + 0.5   Fe and FeO are part of the mineral ash inside the particle. So I am tempted to define the volume fraction of my particle with two User Defined Scalar: Code:
P_USER_REAL(p,0) = 1.0 P_USER_REAL(p,1) = 0.0 Typical laws for the propagation of such species are  where 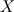 and and  corresponds to the volume fraction of the species and time. corresponds to the volume fraction of the species and time. 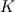 is some coefficient. is some coefficient.Now there is my problem: The species volume fraction inside the particle are 1 for  and 0 for and 0 for  . .The application of these numbers to the above law will lead to zero reaction (because 1 - X = 1 - 1 = 0). This can't be right. The obvious reason? Oxygen! I think I need to include the oxygen into my considerations. The reaction will only take place in an oxygen environment. Remember: The oxygen is part of the allready calculated solution inside the domain. What I am not sure about is the connection between my definition on the particle itself (the User Defined Scalars) and the oxygen concentration in the domain. The volume  -concentration of the system containing my particle and oxygen will be less than 1 ... -concentration of the system containing my particle and oxygen will be less than 1 ...I'm not sure how to approach this. How can I tell if stoichiometric conditions apply? (How do I identify the conditions at all?!) What happens if there is less/more oxygen than needed? Hope I made myself clear. Feel free to answer. Any help is appreciated.
__________________
Schrödingers wife: "What did you do to the cat? It's half dead!" |
|
|
|

|
|
|
|
|
#2 |
|
New Member
sarighulikhan
Join Date: Mar 2014
Posts: 15
Rep Power: 12  |
thats an intersting simulation
can you post ur udf codes here i want to have a look at them |
|
|
|

|
|
|
|
|
#3 |
|
Senior Member
Join Date: Feb 2010
Posts: 164
Rep Power: 17  |
Quote:
|
|
|
|

|
|
|
|
|
#4 |
|
Senior Member
Join Date: Feb 2010
Posts: 164
Rep Power: 17  |
Quote:
|
|
|
|

|
|
 |
|
|
 Similar Threads
Similar Threads
|
||||
| Thread | Thread Starter | Forum | Replies | Last Post |
| A question about solid surface reaction in FLUENT | RAO | FLUENT | 11 | August 12, 2012 03:48 |
| Dispersed Solid particles in carrier gas (DPM) | Frederik | FLUENT | 1 | June 3, 2008 06:13 |
| DPM reaction | Alexandre | FLUENT | 2 | November 12, 2002 02:48 |
| CFX4.3 -build analysis form | Chie Min | CFX | 5 | July 13, 2001 00:19 |
| chemical reaction - decompostition | La S. Hyuck | CFX | 1 | May 23, 2001 01:07 |